
What will be the molarity of the solution in which 0.365 g of HCl gas is dissolved in 100 mL of solution?
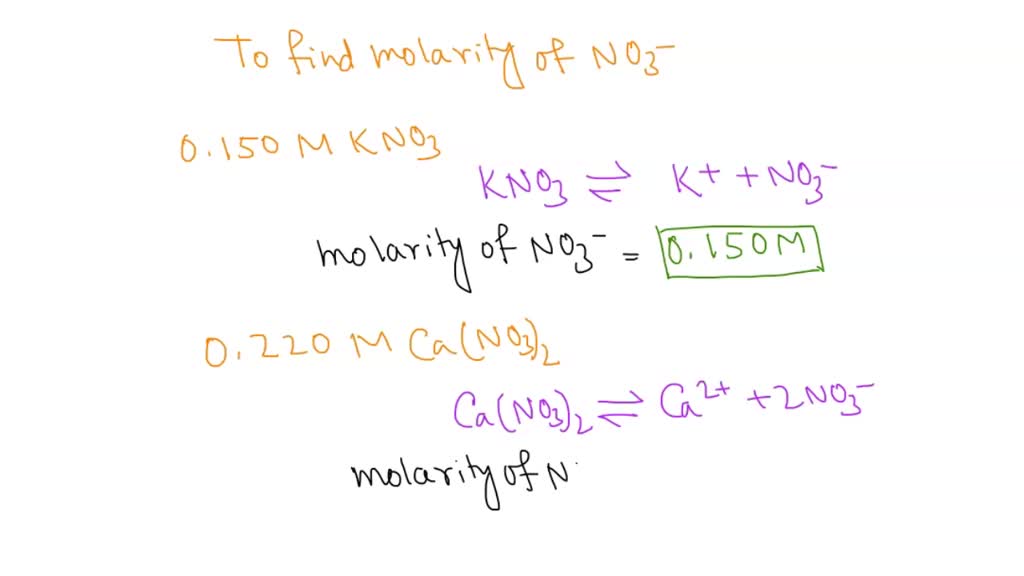
SOLVED: What is the molarity of NO?3 in each solution? 0.150 M KNO3. 0.220 M Ca(NO3)2 0.370 M Al(NO3)3.

SOLVED: What is the molarity of Cl−Cl− in each solution? 0.190 M SrCl2 6.00×10-2M AlCl3 Express your answer with the appropriate units.

Molarity, Molality, Volume & Mass Percent, Mole Fraction & Density - Solution Concentration Problems - YouTube













:max_bytes(150000):strip_icc()/606823-calculate-molarity-of-a-solution-FINAL-5b7d7e15c9e77c0050355d4e.png)



